Event Analysis
Analysis on events that occurred during the initial 6.5 years of the study
The GCKD study employs a comprehensive approach to monitoring adverse events, encompassing all cardiovascular, kidney, and other adverse events, as well as hospitalisations, among study participants. A dedicated team of physicians is responsible for the continuous evaluation and adjudication of these. This guarantees consistent coding.
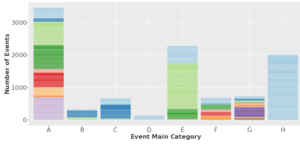
A comprehensive adjudication catalogue focuses on occurrence of events, and comprises eight main event groups: cardiovascular (group A), cerebrovascular (group B), peripheral vascular ( group C), microangiopathy in diabetes (group D), kidney (group E), death (group F), cancer (group G), and hospitalization due to infection (group H). Within these categories, events are classified into subcategories with up to five levels of detail, allowing for the specification of event as detailed as possible.
As part of the CKDNapp junior consortium, a substudy of the GCKD study, interactive plots were generated with R-shiny/Shiny Proxy based on all events achieved in the 6.5 years following inclusion and made accessible via an R-Shiny web application. You can access these plots by clicking on the figure below and get an introduction how to use these in the video tutorial below.
Steinbrenner I, Kotsis F, Kosch R, Meiselbach H, Bärthlein B, Stockmann H, Lipovsek J, Zacharias HU, Altenbuchinger M, Dienemann T, Wytopil M, Bächle H, Sommerer C, Titze S, Weigel A, Weissensteiner H, Schönherr S, Forer L, Kurz NS, Menne J, Schlieper G, Schneider MP, Schäffner E, Kielstein JT, Sitter T, Floege J, Wanner C, Kronenberg F, Köttgen A, Busch M, Krane V, Schmid M, Eckardt KU, Schultheiss UT. Interactive exploration of adverse events and multimorbidity in CKD. Nephrol Dial Transplant. 2024 Apr 25
